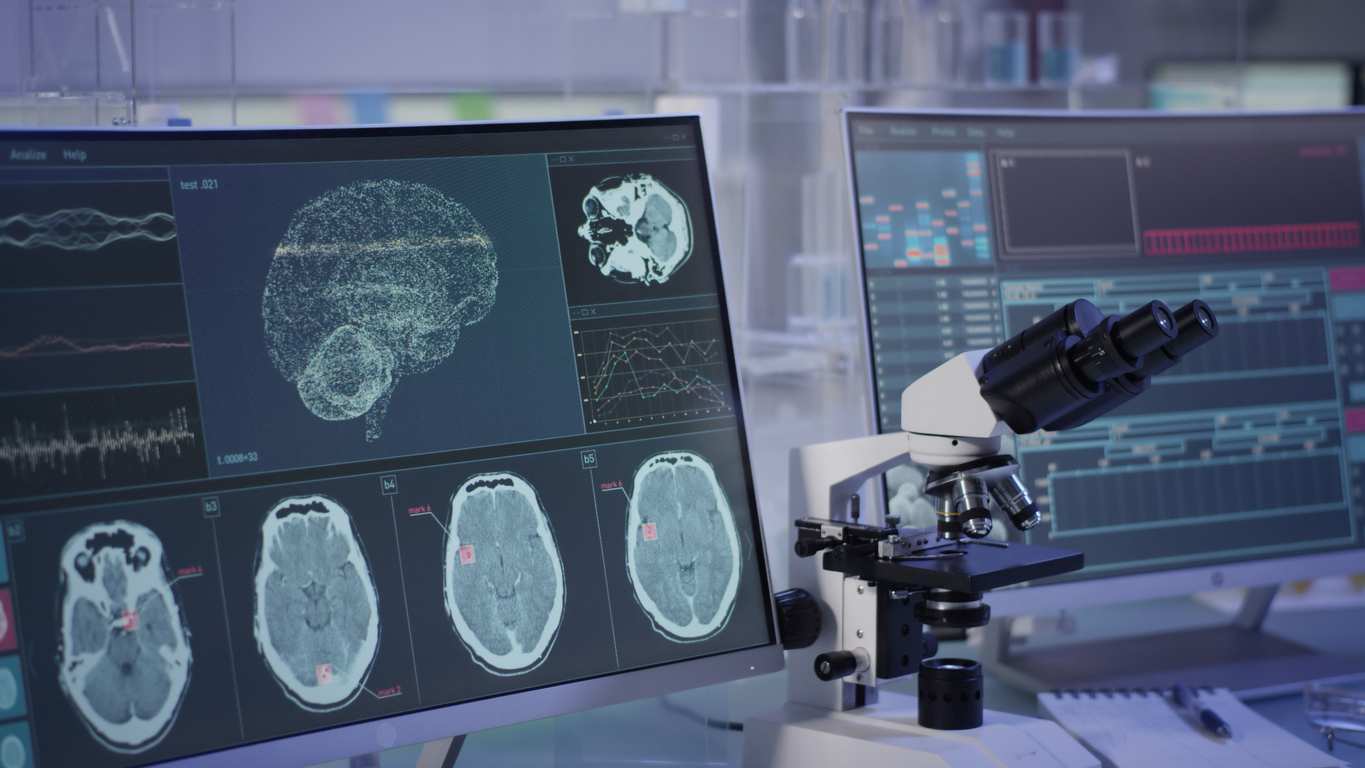
Lilly Receives Positive CHMP Opinion for Emgality
INDIANAPOLIS, Sept. 21, 2018 Lilly Receives Positive CHMP Opinion for EmgalityTM (galcanezumab) for the Prophylaxis of Migraine in Adults Eli Lilly and Company announced today that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has issued a positive opinion for Emgality TM (galcanezumab) for the prophylaxis of migraine in adults who have at least […]
Lilly Receives Positive CHMP Opinion for Emgality Read More »