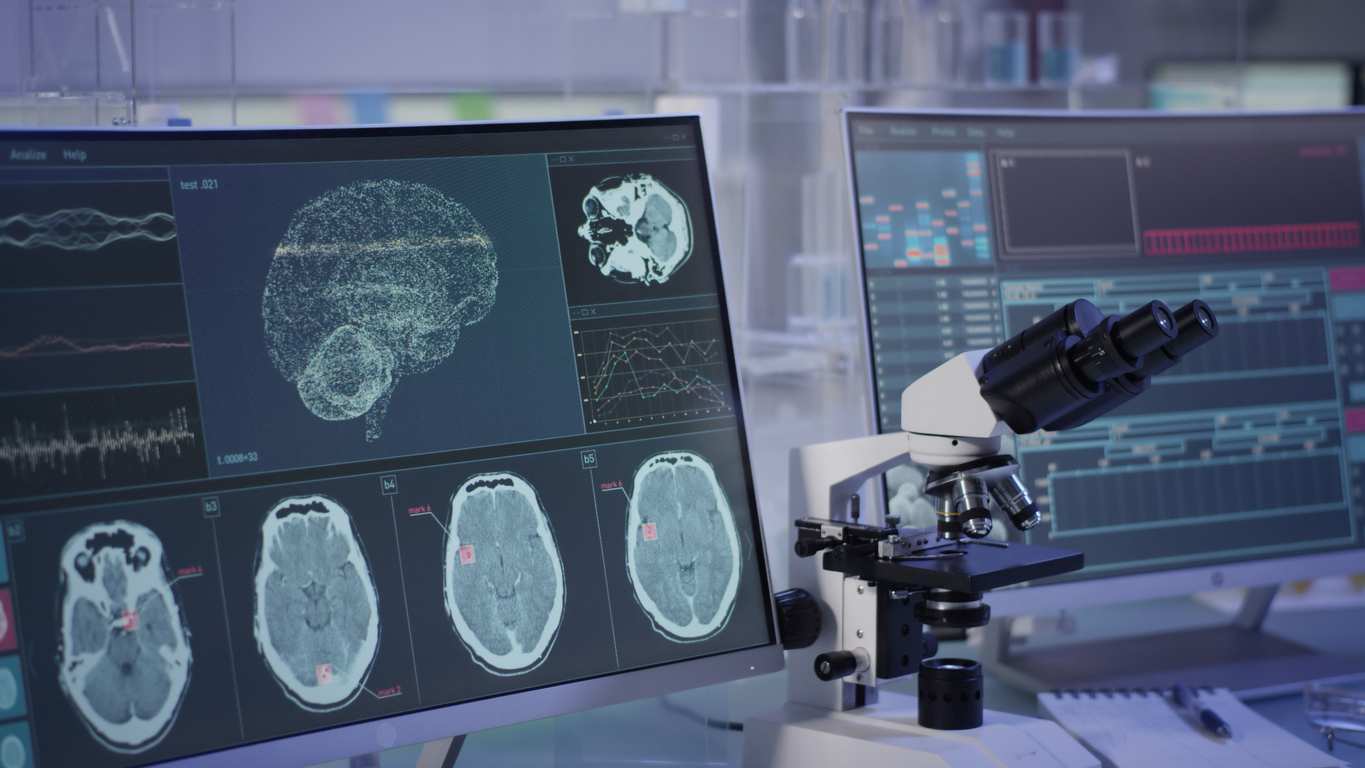
New drugs, decades in the making, are providing relief for migraines.
Nancy Baum Lipsitz remembers the night the pain began. She’d had a glass of white wine with a friend and went to bed with a terrible headache. The next day, she still felt horrible, the beginning of what she called a “rolling tide” of near constant migraines and lower level headaches. For three years she […]
New drugs, decades in the making, are providing relief for migraines. Read More »